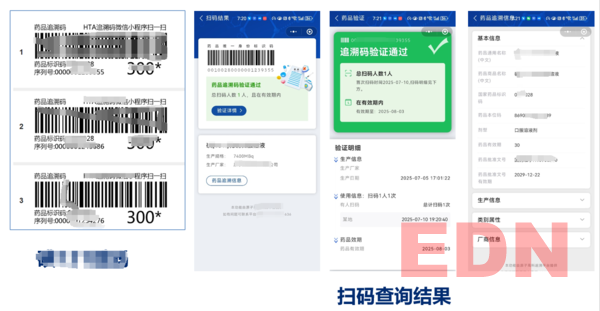
- Radioactive Drug Traceability Platform Successfully Launched
The drug traceability system developed by our company for a pharmaceutical manufacturer was fully built by June 30. It includes an automatic coding system for medicine box production lines, as well as other systems for printing, information association, and uploading of radioactive drug traceability codes (including applications for subsidiaries). The system has passed internal testing.
The platform enables traceability code printing, production data association, and product information upload to medical insurance systems. It also connects with the existing pharmacy platform, iodine interventional platform, and supply chain system, allowing the headquarters and subsidiaries using these systems to achieve automatic information collection. With the input of a batch number, traceability codes can be printed automatically.
Currently, the functions of the traceability code system fully comply with the regulatory requirements of the National Medical Products Administration and the National Healthcare Security Administration, and are capable of coding all products of Gaoke. (The modification process for the automatic medicine box coding system has been completed.)